Industry Background
The medical device industry spans machinery, electronics, plastics, and multiple other sectors. It is a high-tech industry characterized by multidisciplinary integration, knowledge intensity, and capital intensity. Under the momentum of the digital economy, China's medical device industry has grown rapidly, showing strong expansion in both market size and development speed. In particular, the release and implementation of the 14th Five-Year Development Plan for the Medical Equipment Industry, together with the deepening implementation of Unique Device Identification (UDI), have accelerated the industry's digital and intelligent transformation. These changes continue to empower medical device enterprises to enhance productivity, strengthen quality control, increase production capacity, and optimize manufacturing processes.
Management Challenges
Compared with other industries, the medical device industry is characterized by complex process mechanisms, highly planned production, difficult production collaboration, strict batch management requirements, and high medical risk control demands. It is also defined by small batches, multiple varieties, short takt time, and tight delivery schedules. As a project-based discrete manufacturing industry, its complete product assemblies must be decomposed across multiple workshops and warehouses, while on-site operations follow modular production by "functional zones." As a result, enterprises face the following management challenges:
- Incomplete warehouse material data and lack of batch-level traceability lead to low management efficiency.
- Long manufacturing cycles for complete systems such as CR and MR, combined with complex multi-level BOM structures, make kitting difficult and production planning highly challenging.
- Production information remains fragmented, forming isolated data "islands" without end-to-end process connectivity.
- Lack of systematic quality control and error-proofing mechanisms, making full-process traceability impossible.
- Real-time equipment data cannot be obtained, resulting in missing equipment alerts, status monitoring, and OEE analysis.
- Lengthy approval processes for production-related electronic documents, drawings, and records reduce efficiency and impact production progress.
- On-site "improvised" workstation setups ("market stall" style) make production and delivery factors hard to control, leading to difficult production collaboration.
Solution
Morewis provides medical device enterprises with a full value-stream control solution aligned with the industry's "SMT + FATP" business model, enabling the construction of a digital factory. Based on actual management requirements and supported by UDI compliance and pharmaceutical GMP regulations, the solution builds an integrated digital application that combines production, management, and traceability. Using this as a core anchor point, it connects data from other business systems to establish a complete, controlled, and effective central data set across multiple systems, covering the entire lifecycle of medical device manufacturing. The solution embeds production design and process requirements into system workflows, enabling real-time management and monitoring of the production process, end-to-end error-proofing and tracking of materials and processes, and automatic data collection, monitoring, and alerting for production equipment. This allows managers to gain clear visibility into production data, accurately assess quality at every stage, ensure compliance, improve production efficiency, and safeguard product quality.
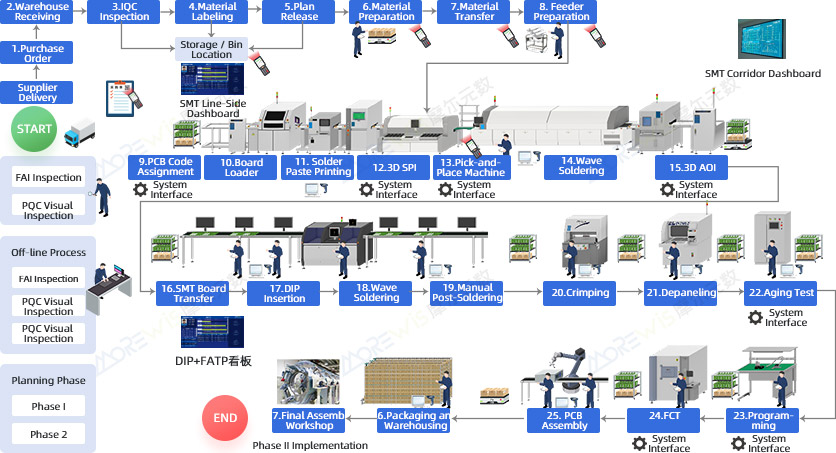
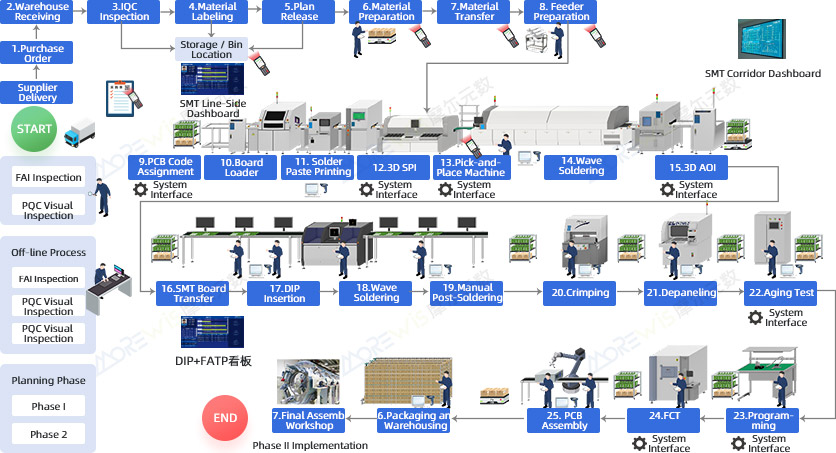
Overview of the Full Value-Stream Control System for "SMT + FATP" in the Medical Device Industry
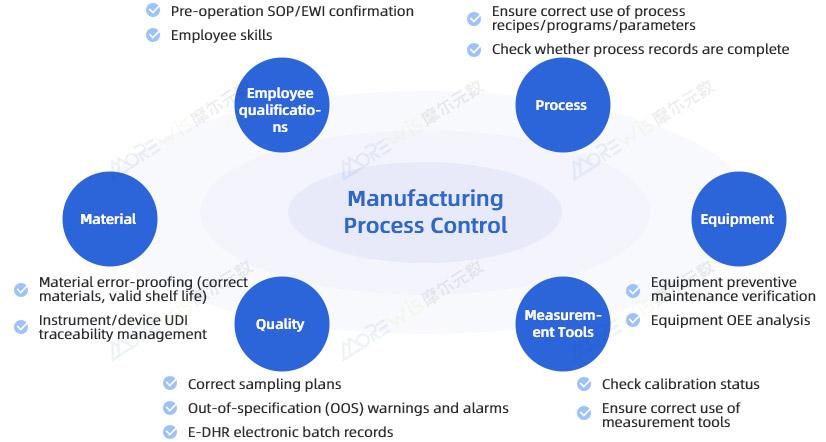
Building a Digital Factory for the Medical Device Industry Through the Full Manufacturing Process
Addressing Core Needs
- Establish a standardized "error-proofing + traceability" management system to enhance regulatory compliance in the medical device industry.
- Automatically collect production process data, archive it, and generate E-DHR electronic batch records, meeting regulatory requirements for precise and efficient traceability.
- Integrate planning, scheduling, material kitting, and production management, reducing production waiting time caused by plan adjustments.
- Enable full transparency and refined management of SMT, DIP + FATP, and final assembly production processes.
- Use hierarchical barcodes to collect, consolidate, and manage data on quality-impacting factors throughout the production process, and build forward- and backward-traceability systems based on the 4M1E framework.
- Achieve comprehensive quality management for IQC, PQC, FQC, and QA, enabling closed-loop control for quality warnings, issue handling, and resolution.
- Implement full TPM-based equipment management, enabling interconnection and interoperability among critical production equipment and inspection devices, along with real-time equipment monitoring, alerts, status visibility, and OEE visualization.
- Realize production transparency through big-data BI, and use data to drive operations and management, and enhance interdepartmental collaboration.
- Obtain real-time information on warehouse materials, consumables, and stencil squeegees, enabling material batch traceability as well as systematic management of incoming inspections, rule setting, and inspection records.
- Provide customized workflow management and electronic signatures, enabling more timely and efficient document review.
Implementation Benefits