Project Background
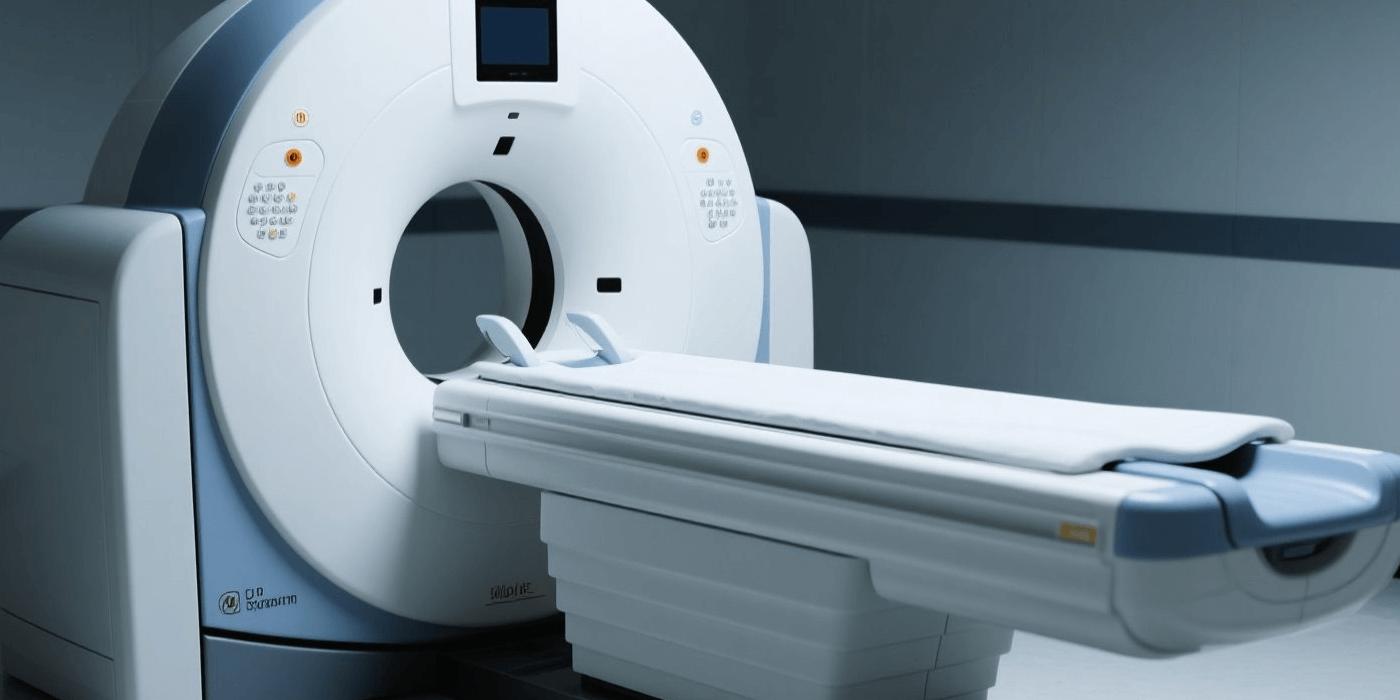
A high-tech enterprise specializing in high-performance medical imaging equipment, radiotherapy products, life-science instruments, and digital and intelligent medical solutions. Its product portfolio—including CT, MR, MI, XR, and RT systems—holds a leading position in the domestic market, making the company a top manufacturer of high-end medical imaging equipment in China.
Business Needs
- High requirements for efficient alignment between planning and production, with customer order changes needing rapid response.
- Lack of real-time visibility into factory production status, with insufficient control over key process quality.
- Numerous components and complex material flows result in low material-management and delivery efficiency.
- Paper-based records cannot support rapid product traceability and recall response.
- Lack of transparency in production-line equipment status and absence of real-time operational feedback.
Implementation Overview
N2.MES Manufacturing Execution System
Covers planning management, material management, process management, production management, quality management, equipment management, tooling and fixture management, warehouse management, big-data analytics, and decision dashboards.
The system supports SMT production lines, DIP insertion lines, and other operating units.
How the Solution Addresses Core User Needs
- Implements end-to-end barcode-based operations, with system monitoring, recording, error-proofing mechanisms, and real-time feedback throughout the entire material-flow process.
- Provides systematic error-proofing and refined collection, recording, and control of 4M1E electronic batch-operation steps.
- Enables integrated management of planning, scheduling, material kitting, and manufacturing, with configurable conditions for material-kitting analysis.
- Collects barcode-based quality-impact factor data at each process level, enabling closed-loop quality traceability management.
- Integrates key equipment to provide comprehensive monitoring and feedback on equipment operating status, capacity, and utilization.
Implementation Benefits
- 80% reduction in material error rate
- 20% improvement in production plan attainment
- 100% enhancement in product traceability
- 30% reduction in warehouse-management costs